Contents
- What is the periodic table?
- How is the A-level periodic table organised?
- Do you need to know the periodic table for A-Level?
- Can I take a periodic table into my A-level chemistry exam?
- Is element 119 possible?
A good understanding of chemistry can lead to careers in areas such as chemical engineering, biomedical engineering, dentistry, medicine, nursing, pharmacology, or veterinary medicine.
Chemistry A-Level was taken by 55,144 students in 2021, an increase of 6.9 per cent from the previous year. It’s often called the “central science” because it links other sciences like physics, astronomy and biology.
While chemistry is central to science, the periodic table is central to chemistry and an essential part of the chemistry A-Level syllabus. 🧪
So, what is the periodic table A-Level students need to know so much about?
What is the periodic table?
It may look like some kind of crazy pixelated steam engine covered in random letters and numbers, but it’s actually the ingredients label for all the matter in the universe! 🪐
Elements are single atoms that can combine with other atoms to make molecules, the stuff that nearly everything you can see, taste, feel, hear, or smell is made of. Starting with hydrogen, which accounts for 90% of all atoms in existence, and going all the way up to Oganesson, one of the most recent to be discovered, the periodic table is a very clever way of organising our knowledge about these elements.
The way the table is arranged is important, and if you know how to read it, you can work out a lot of helpful information about the structure, properties, and behaviour of each element.
This is because locations on the table tell you about the way the electrons are arranged on the outside of atoms. Electrons are what make chemical reactions happen, so if you know how the electrons are organised, you can tell if an atom will react with other elements and what it will do.
How is the A-level periodic table organised?
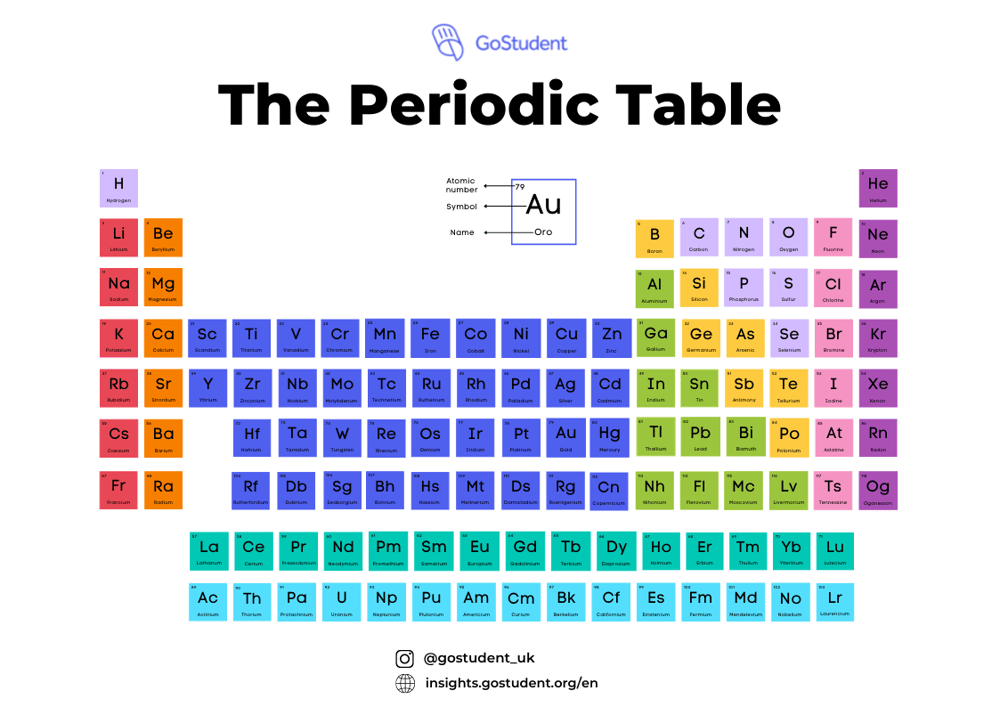
The A-level periodic table is laid out according to the same rules that almost all versions of the table follow. There are 118 boxes, and each box contains the chemical symbol for one element.
For example, let’s look at Carbon, which is represented by the symbol “C”. The number 6 appears in the top left corner, which is the element’s atomic number representing how many protons it has. The relative atomic mass 12 appears at the bottom of the box for Carbon.
Going across the table, the rows are called periods. All the elements on each row have the same number of atomic orbitals, a space around the atom where electrons can be found, and each of the seven rows has a different number of these orbital spaces. ⚛
The first period has one orbital, the second period has two orbitals and so on until you get to seven orbitals on row 7. So you can look at row 4 and know Potassium (K) and Zinc (Zn) have the same number of orbitals, but Sodium (Na), in row 3, has a different number.
Running up and down the table, we have 18 columns known as groups. Elements in the same group have the same number of electrons in their outer shells, which are known as valence electrons. So, for example, in group 1, the Alkali metals and the first column of the periodic table, each element has one electron in the outer shell; group 2, the Alkaline metals, have two electrons and so on.
An element’s position in the periodic table can be used to predict other things like its melting point, atomic radius and electronegativity. It’s called the “periodic table” because certain properties repeat in each period. In fact, the scientist who designed it in 1871, Dmitri Mendeleev, used this pattern of repetition to predict the existence of brand new elements before they were even discovered!
Do you need to know the periodic table for A-Level?
The answer to this depends on what you mean by “know”. It’s not necessary for you to learn the whole A-Level periodic table by heart and be able to draw it from memory. Even most professional chemists can’t do this! 👴
That said, you do need to know how to read it and interpret the information you find there to predict the properties of elements. For example, you should understand why groups and periods are arranged the way they are, what their electron configurations look like and know which part of the table contains things like noble gases, non-metals, transition metals or halogens.
The best way to learn your way around the A-Level periodic table is to use it regularly. Test yourself by trying to read and interpret different parts of it, then check your answers on a great interactive site like Periodictable.com or Ptable.com, which have shed loads of info on all the elements.
Can I take a periodic table into my A-level chemistry exam?
Well, there’s good news and bad news here. First, we’ll give you the bad news: no, you can’t take your A-Level periodic table into the exam. If they let candidates bring a personal copy of the table into the exam, cheaters could add all sorts of useful crib notes in invisible ink!
So, do you get a periodic table in chemistry A level?
Now the good news: your friendly neighbourhood exam board will include a fresh new copy of the very latest A-Level periodic table free with every exam paper! How generous is that? 🎁
Is element 119 possible?
There are 118 elements on the current periodic table, so you might be wondering if it’s possible for there to be more.
The answer is yes, up to a point. Between 94 and 98 of the elements on the table occur naturally in the universe. The others, including the last three to be discovered, Moscovium, Tennessine, and Oganesson, have to be made artificially in a lab or nuclear accelerator.
But these synthetic elements are tough to make and typically only exist for a very short time.
Scientists around the world are racing each other to create element 119, but it could be decades before they succeed! That gives you plenty of time to study hard for your A-Level chemistry, finish university and start your PhD! Perhaps, one day you’ll be the chemist that finds it?
If you need help finding your way around the periodic table or telling your isotopes from your allotropes, GoStudent’s professional chemistry tutors are ready and waiting to give you expert help and advice. Hit this link to check out our free trial today!